The US Food and Drug Administration (FDA) has issued a warning to dog owners regarding flea and tick medications due to reports of serious adverse reactions. While this signals a growing acknowledgment of the potential dangers associated with these products, the extent of the FDA’s attention to the severity of these risks is still being evaluated. The agency’s recent report highlights concerns over neurological issues in pets linked to these common treatments.
Understanding the FDA’s Warning
Last month, the FDA announced that flea and tick medications have been associated with neurological problems in pets. The most frequently reported symptoms include muscle tremors, ataxia (loss of coordination), and seizures. This acknowledgment comes after numerous reports from concerned pet owners and veterinarians.
The side effects detailed by the FDA for these drugs encompass a range of concerning conditions:
- Tremors
- Seizures
- Ataxia
- Vomiting
- Diarrhea
- Loss of appetite
- Skin irritations
- Lethargy
The specific products implicated in the FDA’s report are Bravecto, Nexgard, Simparica, and Credelio (which received FDA approval in 2018). A common element among these medications is the presence of an ingredient called isoxazoline.
How Isoxazoline Flea and Tick Medications Work
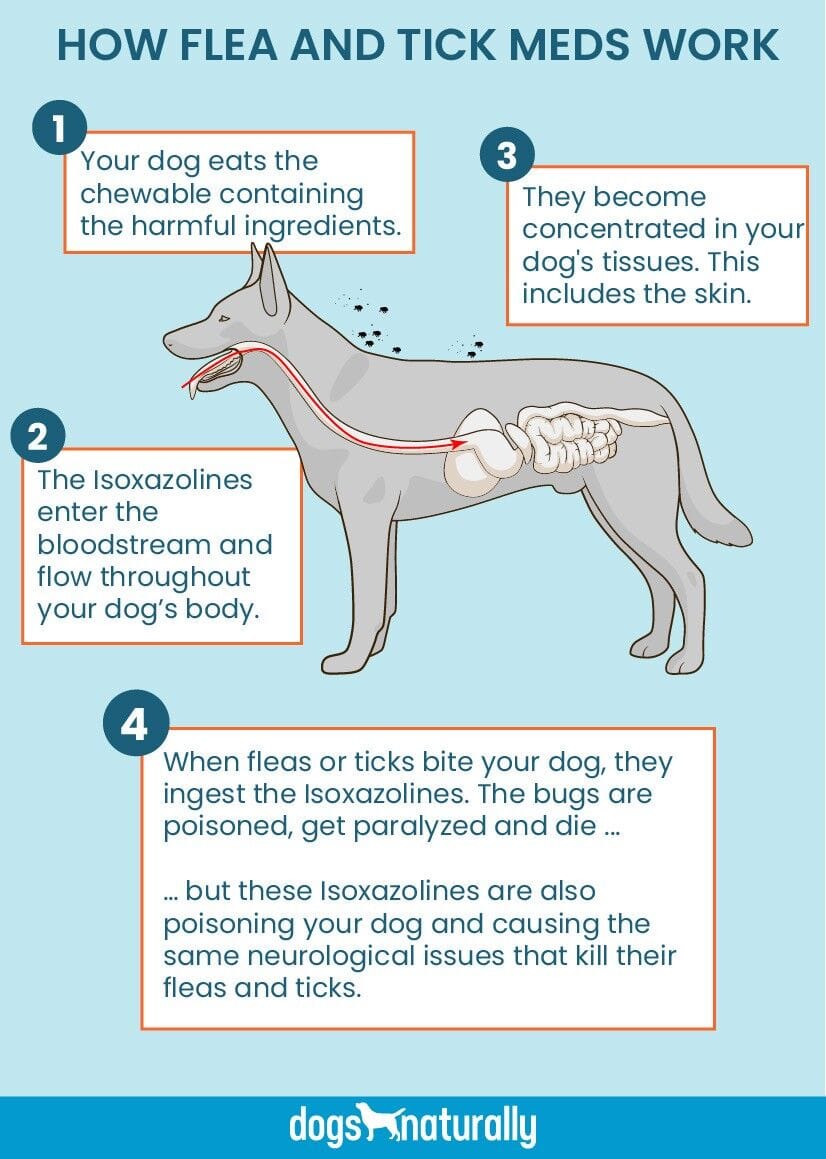
Isoxazolines function as non-competitive GABA (gamma-aminobutyric acid) receptor antagonists. Essentially, they bind to chloride channels within fleas and ticks, disrupting nerve signals and leading to paralysis and death of the pests. When administered to dogs, these drugs are absorbed into the bloodstream, affecting the entire system. Fleas and ticks ingest the chemical when feeding on the dog’s blood, which then paralyzes and kills them.
Potential Dangers for Dogs
The fundamental issue with using systemic poisons to eliminate fleas and ticks is that it necessitates exposing the host—your dog—to the toxic substance. The underlying assumption is that a dog’s larger size compared to a flea means a small dose of poison will not cause harm. While this may hold true in many instances, a critical question remains unanswered: what are the long-term effects of administering a small dose of poison monthly for several years?
Current safety studies for these drugs are often limited to a few months, raising concerns about their long-term safety profile. Furthermore, the FDA’s stance that tremors and ataxia are not necessarily cause for alarm is debated by experts. These symptoms could indicate that dogs are indeed being poisoned alongside the pests, albeit at a slower rate, and are experiencing neurological issues similar to those affecting the fleas and ticks. Consequently, every dog may be at risk.
Veterinarian Perspectives on Flea and Tick Medications
Veterinarians have expressed significant concerns regarding the safety and long-term effects of these medications.
Dr. Tamara Hebbler stated, “This is no surprise. Every known insecticide/pesticide chemical has been shown to have severe neurological side-effects.” With 20 years of practice, she emphasizes asking about insecticides (oral, topical, and environmental) for seizure patients. She believes that keeping up with insecticide resistance mutations and toxicity buildup is a significant challenge, advocating for safe, natural alternatives and empowering pet owners with knowledge.
Dr. Josie Beug noted the public’s assumption that the FDA thoroughly tests new products for safety. She advises a “wait and see” approach for a couple of years after a new pharmaceutical’s release, as safety testing is typically conducted over only a few months, while products are used monthly for years. Dr. Beug highlights that “All of the flea and tick preventatives are toxic, they’re made to kill insects after all.” She recounted a case of a one-year-old dog who experienced severe seizures for the first time three days after receiving Bravecto. Despite extensive veterinary care, including high doses of anticonvulsants and even CBD oil, the dog continued to suffer from cluster seizures. The owner was unaware of the potential link to Bravecto and administered it a second time. Dr. Beug also points out the difficulty in definitively proving that a specific toxin caused the seizures, which allows manufacturers to avoid repercussions. She mentioned five other cases of dogs and cats with neurological and ocular signs coinciding with heavy organophosphate spraying for mosquito control, illustrating the challenge of proving cause and effect. She urges pet owners to stay vigilant, research products, and report any adverse reactions to the FDA.
Dr. Deva Khalsa, with 40 years of veterinary experience, believes that “products that kill fleas and ticks aren’t healthy.” She explains that oral or topical products concentrate in a dog’s tissues and bloodstream, lasting for one to three months. Isoxazolines bind to specific cell channels, blocking neuronal signal transmission in pests. While intended to be more selective for fleas and ticks than mammals, neurological signs in dogs indicate that these chemicals are also affecting their nervous systems. Dr. Khalsa points out that the FDA’s decision to add a warning label is a result of numerous reports, but many adverse reactions, including death, may go unreported. She speculates that many pets experience milder, less obvious reactions such as weakness, dizziness, or slight imbalance, which are often overlooked. Vomiting is also cited as a common side effect. Dr. Khalsa’s opinion is firm: “There’s no pharmaceutical product that kills fleas and ticks without harming your dog.” She mentions that even pyrethrin products have been associated with a high incidence of death due to the large quantities required for effectiveness. If a pet is not prone to fleas and ticks, she advises against using any such products. She has observed veterinary medicine becoming increasingly corporate, with vets administering unnecessary vaccines and dispensing “toxic products like candy.”
Natural Alternatives and Vigilance
Veterinarians and advocates have long been raising awareness about the risks associated with conventional flea and tick medications. While the FDA’s warning is a step towards acknowledging these concerns, there is a consensus that more robust safety measures and greater transparency are needed. Many experts recommend exploring natural alternatives that have proven effective without posing significant risks to pets.
For pet owners concerned about the potential dangers of isoxazoline-based flea and tick treatments, exploring natural prevention methods and consulting with holistic veterinarians can provide safer, equally effective solutions. Staying informed and advocating for your pet’s health is crucial in navigating the options available for pest control.
The FDA is working with manufacturers to update labels, highlighting the neurological events associated with isoxazoline products. This move, while a positive development, underscores the importance of pet owners being informed consumers, carefully researching products, and reporting any adverse reactions to ensure the well-being of their beloved companions.