Millions of children globally face rare and debilitating developmental disorders, and unlocking their mysteries is a monumental challenge. Fortunately, our beloved canine companions are emerging as invaluable allies in this quest. Dogs, with their unique genetic makeup and shared environments with humans, serve as remarkable physiological models for these conditions, offering profound insights into Rare Genetic Disorders In Dogs and their human counterparts.
The journey of dog domestication, stretching back to the Eurasian gray wolf, has led to an incredible diversity of breeds, each with distinct traits. This rapid evolution, driven by selective breeding over the last 200 years, has resulted in breeds acting as isolated populations. While this has given us a rich tapestry of canine appearances and behaviors, it has also inadvertently concentrated specific genetic conditions within breeds. This genetic architecture, characterized by short and long-range linkage disequilibrium, makes dogs exceptionally powerful for gene discovery in disease, morphology, and behavior.
The dog genome shares more alignment with the human genome than with the mouse, boasting a similar number of genes, many of which are close orthologues. This biological proximity, combined with the fact that pet dogs share their owners’ lifestyles and environmental exposures, makes them highly relevant models. Hundreds of common canine conditions—such as diabetes, cancers, epilepsies, and autoimmune diseases—mirror human illnesses. Crucially, a significant number of rare monogenic diseases in dogs also have direct equivalents in humans, as documented in the Online Mendelian Inheritance in Animals (OMIA) database. Understanding [common disease of dog](https://dogcarestory.com/common-disease-of-dog/) helps us appreciate the broader spectrum of canine health challenges.
Moreover, the availability of well-recorded genealogical data through kennel clubs, like the Finnish Kennel Club, provides invaluable resources for genetic studies. Combined with detailed veterinary records, this data ensures the reliability and success of research efforts. Institutions like the comparative genetic program in Finland, with its extensive canine biobank of over 60,000 samples from more than 300 breeds, actively leverage these resources. Their work has led to numerous gene discoveries, particularly in developmental disorders that serve as models for human pediatric conditions. These breakthroughs pave the way for preclinical trials and new therapeutic interventions, such as recombinant protein replacement for X-linked hypohidrotic ectodermal dysplasia (XLHED) and gene therapies for hemophilia A and retinal degeneration.
Why Dogs Are Exceptional Models for Rare Genetic Disorders
Dogs offer a unique combination of clinical, genetic, physiological, and environmental similarities to humans, making them excellent comparative models. This enables scientists to better understand and treat both canine and human disorders. Our recent research, for instance, identified three new developmental disorders and their causative genes in dogs, providing highly relevant models for rare human diseases. These spontaneous canine conditions closely resemble their human counterparts, offering physiologically relevant systems to unravel poorly understood gene functions and molecular pathologies, ultimately leading to innovative therapeutic strategies.
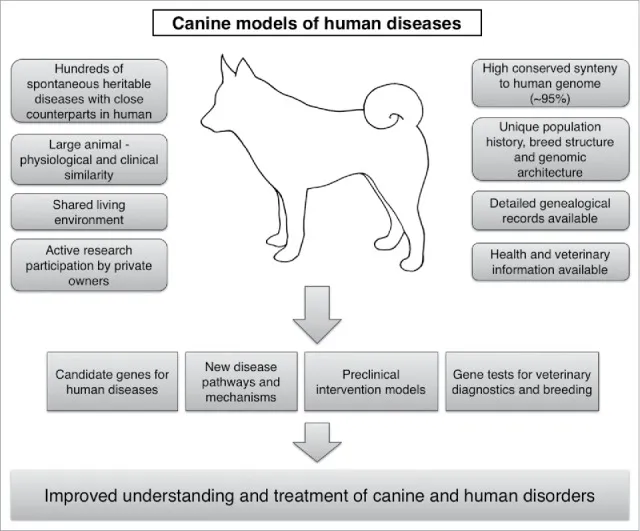 A diagram illustrating how various clinical, genetic, physiological, and environmental characteristics in dogs make them excellent comparative models for human disorders.
A diagram illustrating how various clinical, genetic, physiological, and environmental characteristics in dogs make them excellent comparative models for human disorders.
Canine Model of Infantile Cortical Hyperostosis (Caffey Disease)
Craniomandibular osteopathy (CMO) is a painful, self-limiting condition in young dogs characterized by jaw swelling, leading to difficulties in eating and opening the mouth. It is recognized as the canine equivalent of human infantile cortical hyperostosis, also known as Caffey disease. We recently discovered a splicing defect in the SLC37A2 gene in dogs with CMO, proposing it as a strong candidate for mutation screening in human Caffey patients.
This discovery is particularly significant because the molecular cause of Caffey disease remains unknown in many human cases, and an early molecular diagnosis could prevent invasive procedures. Furthermore, it offers new insights into the disease’s etiology, as the exact mechanism by which previously identified variants contribute to this self-limiting hyperostosis is still unclear. The SLC37A2 gene, part of the SLC37 family of ER-associated glucose-phosphate transporters, is highly expressed in bone marrow and hematopoietic cell lineages like osteoclasts and macrophages, suggesting a crucial role in glucose homeostasis during bone formation.
Our hypothesis is that an impaired function of SLC37A2 disrupts the proper glucose supply to osteoclasts, reducing their activity. This imbalance between bone-building (osteoblastic) and bone-resorbing (osteoclastic) functions during bone development could lead to hyperostosis. Interestingly, SLC37A2 has also been identified as a primary target for vitamin D, which could potentially serve as a therapeutic booster to alleviate clinical signs in affected dogs. This canine study not only pinpointed a novel gene linked to Caffey disease but also shed light on the physiological function of SLC37A2, opening new avenues for understanding infantile swelling diseases related to impaired glucose homeostasis during bone development. These affected dogs now provide unique resources for further research into these hypotheses.
Canine Model of Van den Ende-Gupta Syndrome (VDEGS)
Our second example highlights how gene discovery helps define an illness and establish a relevant model. We identified a severe truncating mutation in the SCARF2 gene in Wire Fox Terriers suffering from an undiagnosed developmental syndrome marked by severe mandibular prognathia (an abnormally protruding lower jaw) and other skeletal abnormalities. This canine condition closely mirrors human van den Ende-Gupta syndrome (VDEGS), which is characterized by various craniofacial and skeletal anomalies, including distinctive facial features and limb abnormalities.
Many similarities exist between human and canine VDEGS patients, such as hypoplastic maxilla (underdeveloped upper jaw), dislocated radial head, patellar dislocation, deviated nasal septum, and small eyes. The discovery of the SCARF2 mutation in dogs has thus established a definitive canine diagnosis and a valuable model for human VDEGS. This is especially important given the scarcity of human patients and the absence of a transgenic mouse model, making affected dogs a novel resource for understanding SCARF2 functions and molecular pathology. Since some VDEGS dogs live beyond 10 years, they could even serve as preclinical models for long-term studies. [my dog has digestive problems](https://dogcarestory.com/my-dog-has-digestive-problems/) can also be a complex issue, requiring careful observation, much like understanding rare genetic disorders.
SCARF2 is a poorly understood member of the scavenger receptor type F family, a single-pass transmembrane protein with homology to calmodulin-like Ca2+-binding proteins. While it’s known to be expressed in disease-relevant tissues during mouse embryonic development, its exact function remains unknown. Detailed histopathological characterization of canine VDEGS is crucial to unraveling SCARF2 functions, its regulation, and related cellular pathways. This understanding will improve our grasp of disease mechanisms and potentially lead to new therapeutic hypotheses. The established canine model offers powerful new resources to achieve these aims.
Canine Model of Raine Syndrome
Mutations in the FAM20C gene are associated with human autosomal recessive osteosclerotic bone dysplasia, known as Raine syndrome. This rare condition, with less than 40 reported cases, shows variable severity and clinical presentation. Typical features include hypophosphatemia, abnormal and hypomineralized teeth, craniofacial anomalies such as exophthalmos (bulging eyes), midface hypoplasia, microcephaly (small head), cleft palate, gingival hyperplasia (gum overgrowth), generalized osteosclerosis (abnormally dense bones), and intracerebral calcifications.
In a family of affected dogs, we identified a recessive missense variant in the kinase domain of the FAM20C gene. These dogs suffered from severe dental wear and tooth loss. Their clinical findings were primarily limited to severe hypomineralization of teeth, leading to extensive wear and inflammation. Notably, we did not observe some of the typical gross changes seen in human Raine patients, such as hypophosphatemia and distinct craniofacial anomalies. This observation from our canine study raises an important question: could a more limited human phenotype of Raine syndrome exist, and should it be specifically targeted for mutation screening? Given the significant clinical heterogeneity among human patients, more detailed radiographic analyses in dogs might reveal subtle changes beyond the dental phenotype. [my dog has stomach problems](https://dogcarestory.com/my-dog-has-stomach-problems/) can also be a symptom of various underlying health issues, much like the varied manifestations of genetic disorders.
Studying different species can expand our understanding of the clinical heterogeneity of diseases and provide crucial insights into genotype-phenotype correlations. For instance, Fam20c-deficient mice exhibit a prominent dental phenotype, similar to what we observed in our FAM20C-deficient dogs and human patients. Unlike rodents, dogs have a dental physiology closer to humans, with both deciduous (baby) and permanent dentitions. This makes them a relevant preclinical model for this rare human bone disease, especially concerning dental aspects.
Conclusion
The evidence overwhelmingly demonstrates that dogs harbor numerous clinically and genetically similar counterparts to human rare diseases. A deeper characterization and utilization of these canine models hold immense potential, not only for developing new therapies for rare human diseases but also for gaining a more comprehensive understanding of the molecular pathophysiology of these conditions in general. The accelerating pace of gene discoveries in dogs will continue to establish exciting new models for human diseases. Actively encouraging pet owners to participate in preclinical trials could provide a direct pathway to validate human treatment approaches, while simultaneously benefiting canine health and welfare. The full preclinical potential of canine models is still largely untapped, representing a frontier in both veterinary and human medicine.
