Canine Hip Dysplasia (CHD) stands as one of the most prevalent and challenging inherited orthopedic conditions affecting dogs worldwide. For any dog owner or aspiring breeder, understanding the intricate Hip Dysplasia In Dogs Genetics is paramount. This complex condition, influenced by a blend of genetic predisposition and environmental factors, can lead to debilitating pain and significantly impact a dog’s quality of life. At “Dog Care Story,” we aim to demystify CHD, providing you with high-quality, actionable insights to help you raise healthy, happy canine companions.
CHD was first recognized in dogs in 1935 in the USA, and since then, veterinarians and geneticists have been working tirelessly to understand its causes and find effective prevention strategies. It’s a polygenic trait, meaning multiple genes contribute to its expression, making the genetic puzzle particularly intricate. While the phenotypic expression – what we see physically – is often modified by external factors, the underlying genetic quality of a dog plays a critical role in whether it develops this condition and how severely it manifests. This deep dive into the genetics of hip dysplasia in dogs will explore everything from its basic inheritance patterns to advanced diagnostic tools and future prospects in breeding.
What is Canine Hip Dysplasia (CHD)?
Canine Hip Dysplasia is a developmental orthopedic condition characterized by the abnormal formation of the hip joint. In a healthy hip, the ball (femoral head) of the thigh bone fits snugly into the socket (acetabulum) of the pelvis. In dysplastic hips, this fit is loose and unstable, leading to excessive movement, grinding, and eventual degeneration of the joint, culminating in debilitating osteoarthritis.
This condition is particularly common in large and giant breeds, though it can affect dogs of all sizes. Breeds like Labrador Retrievers, German Shepherds, Golden Retrievers, Rottweilers, and Saint Bernards are frequently cited for their higher prevalence of CHD. While some dogs may show mild or no clinical signs, others suffer from severe pain requiring extensive medical or surgical intervention. Understanding the genetic components is crucial because, unlike many diseases with a single identifiable cause, CHD’s polygenic nature means its inheritance pattern is more complex than a simple dominant or recessive trait.
The Genetic Roots of Hip Dysplasia: A Polygenic Challenge
At the heart of hip dysplasia in dogs genetics lies its polygenic inheritance pattern. This means that multiple genes, each contributing a small additive effect, work together to determine a dog’s susceptibility to CHD. This contrasts with monogenic diseases, which are caused by a single gene mutation and are often easier to trace and predict. The complexity of polygenic inheritance is why breeding out CHD has been a slow and challenging process.
Heritability and Genetic Complexity
The heritability of CHD, which is the proportion of phenotypic variation (observed traits) attributable to genetic variation, ranges widely from 0.1 to 0.83. This variation depends on several factors, including the specific dog population (pedigree), the methods used to calculate heritability, and the hip phenotypes analyzed (e.g., hip joint laxity versus signs of degenerative joint disease). A higher heritability estimate indicates that genetics play a more significant role in the expression of the trait. For instance, passive hip laxity, a major risk factor for CHD, has shown high heritability in certain breeds, suggesting that genetic selection against this specific trait could be highly effective.
Despite this genetic influence, CHD is generally not considered a congenital disease (present at birth). Puppies are typically born with normal, congruent hip joints. The critical period for hip joint development is believed to be the first 60 days of a puppy’s life. During this time, the depth of the acetabular cavity and the conformation of the proximal femoral head and neck are highly susceptible to remodeling based on stress loading and other factors. This developmental aspect highlights how both nature (genetics) and nurture (environment) conspire to shape a dog’s hip health.
Environmental Influences on Genetic Expression
While genetics lay the groundwork, environmental factors can significantly modify the expression and severity of CHD. Key environmental influences include:
- Nutrition and Body Weight: Overfeeding and rapid growth in puppies, especially large breeds, can put excessive stress on developing joints, exacerbating genetic predispositions. Obesity in adult dogs also worsens joint stress and pain.
- Exercise and Activity Levels: Inappropriate or excessive exercise, particularly high-impact activities, during critical growth phases can negatively affect hip development. Conversely, controlled, moderate activity helps build muscle to support the joint.
- Sex and Age: These factors can also influence the manifestation and progression of the disease, although their direct impact on the genetic expression is less understood compared to nutrition and exercise.
- Surface and Confinement: Slippery surfaces or prolonged confinement can also hinder proper musculoskeletal development.
It’s crucial to understand that a dog with excellent genetics for hip health can still develop some degree of dysplasia if subjected to adverse environmental conditions. Conversely, a dog with a genetic predisposition might have a less severe outcome with optimal care. This interplay underscores why breeding programs must consider both genetic selection and proper management of puppies to effectively reduce the incidence of CHD.
Recognizing the Signs: Epidemiology and Clinical Manifestations
CHD remains a common problem, with prevalence exceeding 50% in some large and giant breeds. What’s interesting is that the clinical presentation of the disease often doesn’t correlate directly with the radiographic changes. A dog with severe radiographic signs might show minimal lameness, while another with less obvious changes on X-ray could be in significant pain.
Clinical signs are typically more evident in two age groups:
- Young dogs (under 1 year of age): Symptoms often stem from hip instability. Owners might observe a slight to moderate lameness, a “bunny hopping” gait (using both hind legs simultaneously when running), difficulty rising, or reluctance to climb stairs.
- Adult dogs: Chronic pain from osteoarthritis is the primary driver of symptoms. Over time, the body attempts to stabilize the loose joint through fibrosis and thickening of the joint capsule, which can temporarily mask clinical signs in middle-aged animals. However, as osteoarthritis progresses, pain and functional limitations typically return, often more severely.
Early detection is challenging because there are no pathognomonic (unique and unmistakable) clinical signs specific to CHD alone. Therefore, a comprehensive diagnostic approach involving physical examination and imaging is essential. [internal_links]
Diagnosing Hip Dysplasia: From Physical Exams to Advanced Imaging
Accurate diagnosis is fundamental for both treating affected dogs and for informed breeding decisions. Diagnostic methods fall into two main categories: those evaluating hip joint laxity (HJL), primarily for young animals, and those detecting signs of osteoarthritis (DJD) in older dogs.
Physical Examination Techniques
The most widely used physical maneuver in veterinary medicine for diagnosing HJL in young dogs (4–12 months of age) is the Ortolani test. This test, typically performed under sedation or anesthesia, involves specific manipulation of the hip joint. The veterinarian applies proximal force to the stifle joint and slowly abducts the limb. In dysplastic hips, the femoral head may be displaced dorsally and then reduce back into the acetabulum, producing a palpable and/or audible “clunk” – a positive Ortolani sign. This indicates abnormal hip joint laxity, a key predictor of future CHD.
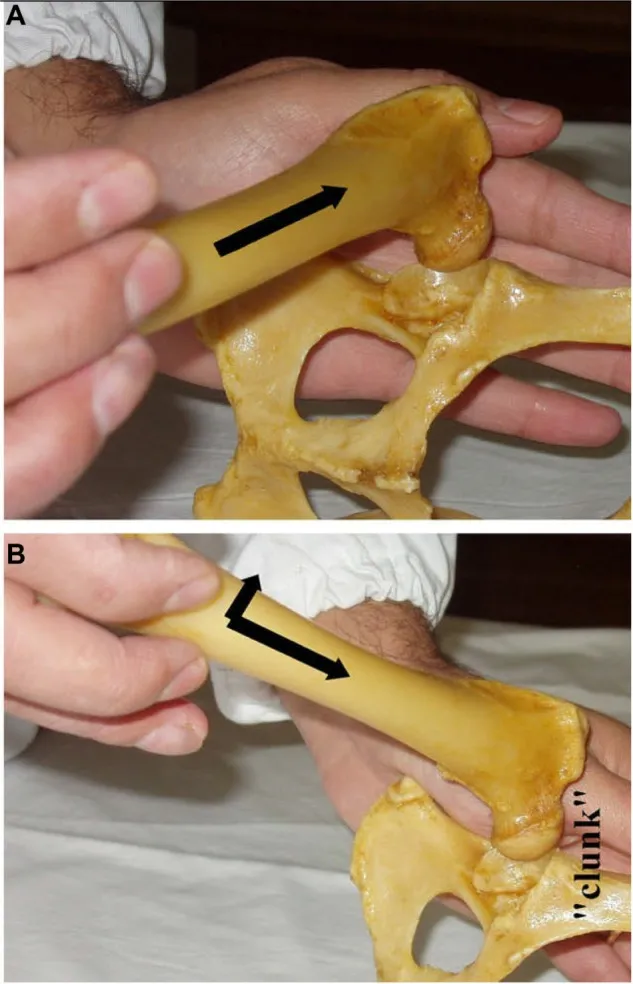 Veterinarian performing the Ortolani test on a dog in lateral recumbency to check for canine hip dysplasia (CHD) and hip joint laxity, showing the displacement and reduction of the femoral head.
Veterinarian performing the Ortolani test on a dog in lateral recumbency to check for canine hip dysplasia (CHD) and hip joint laxity, showing the displacement and reduction of the femoral head.
While Ortolani is valuable, it has limitations. Advanced stages of CHD with severe acetabular rim destruction or very young puppies (under 4 months) with inadequate acetabular ossification can yield false negatives. Other tests like Barlow’s and Barden tests exist for younger puppies, but their accuracy is less certain.
The Role of Diagnostic Imaging
Radiography remains the cornerstone for the definitive diagnosis of CHD. Since its first description, various radiographic views have been developed for genetic screening and for diagnosing and treating clinical cases. All these techniques usually require anesthesia or heavy sedation to ensure accurate positioning and to allow for the assessment of passive HJL without muscle resistance.
Imaging techniques generally focus on two aspects:
- Radiographic estimation of Hip Joint Laxity (HJL): Methods like PennHIP (University of Pennsylvania Hip Improvement Program) are designed to quantify HJL. PennHIP is notable for its early diagnostic capabilities, accurately assessing dogs as young as 16 weeks. It requires three specific radiographic views (hip-extended, compression, and distraction) and measures a “distraction index” (DI), which reflects the degree of femoral head displacement from the acetabulum. A lower DI indicates a tighter, healthier hip.
- Radiographic evaluation of Degenerative Joint Disease (DJD): The Standard Ventrodorsal Hip-Extended View (SVDV) is universally used, typically for dogs aged 1 year (Fédération Cynologique Internationale – FCI) or 2 years (Orthopedic Foundation for Animals – OFA). This view assesses signs of osteoarthritis, joint congruence, and remodeling of the femoral head and acetabulum. Various international scoring systems (FCI, OFA, British Veterinary Association/Kennel Club – BVA/KC) grade hip quality based on these radiographic findings, ranging from excellent to severe dysplasia.
It’s important to note a significant limitation of the SVDV: the hip-extended positioning can artificially tighten the joint capsule, potentially underestimating true hip laxity. This is why methods like PennHIP, which specifically measure passive laxity, are considered more predictive of future CHD development. Despite the subjectivity inherent in scoring systems, these radiographic screenings have been invaluable for selecting breeding stock and monitoring hip health trends over decades.
Beyond X-rays: Other Imaging Modalities
While radiography is primary, other advanced imaging techniques contribute to understanding CHD:
- Ultrasonography: Although the reference for human neonates with hip dysplasia, its use in puppies for definitive CHD confirmation is limited after 8 weeks due to ossification masking acetabular details. Dynamic ultrasonography can quantify HJL in very young puppies.
- Magnetic Resonance Imaging (MRI): Detected increased synovial fluid volumes in hip joints of 8-week-old puppies correlated with later HJL and CHD, offering insights into early changes.
- Computed Tomography (CT): Provides detailed 3D images, allowing confident evaluation of HJL and osseous acetabular structure.
[internal_links]
Managing CHD: Prevention and Treatment Strategies
Given that there’s no single ideal cure for CHD, management focuses on a combination of preventive measures, conservative treatments for mild cases, and surgical options for more severe conditions. For dog owners, understanding these strategies is key to improving their pet’s quality of life.
Conservative Management: Diet, Exercise, and Medication
Preventive conservative management is often recommended for young dogs at risk or those with mild signs:
- Weight Management and Nutrition: Limiting food consumption and preventing obesity are crucial. Maintaining a lean body weight reduces stress on the hip joints. Specially formulated large breed puppy foods promote slower, controlled growth.
- Controlled Activity: Regulated, low-impact exercise helps develop strong muscular tissues to support the hip joint without causing undue stress. High-impact activities should be avoided, especially during growth.
- Disease-Modifying Osteoarthritis Drugs (DMOADs): Injections of DMOADs can help retard cartilage breakdown and promote cartilage matrix synthesis, reducing pain and inflammation.
- Analgesics and Anti-inflammatories: Non-steroidal anti-inflammatory drugs (NSAIDs) and other pain medications manage pain and lameness. However, these are generally for short-term use due to potential side effects, and their ability to prevent osteoarthritis progression is limited.
The effectiveness of conservative treatment for preventing disease progression is often questioned, highlighting the importance of early diagnosis and, ideally, genetic prevention.
Surgical Interventions: When and Why
When conservative management isn’t sufficient, various surgical options are available:
- Juvenile Pubic Symphysiodesis (JPS): A minimally invasive procedure performed on puppies 14–20 weeks old, ideally around 15 weeks. It involves inducing premature closure of the pubic growth plate, leading to modified pelvic growth that increases acetabular coverage of the femoral head and reduces subluxation. JPS is most effective in puppies with slight to moderate signs and good hip conformation.
- Triple Pelvic Osteotomy (TPO): A more invasive surgery for dogs aged 5–12 months with minimal or no DJD signs but significant hip laxity. The pelvis is cut in three places, rotated to improve acetabular coverage, and stabilized with a plate. TPO is more effective when performed before 7 months of age. Hips with existing osteoarthritis or very high laxity have less favorable outcomes.
- Femoral Head and Neck Excision (FHO): This salvage procedure removes the femoral head and neck, eliminating bone-on-bone pain. A “false joint” forms from fibrous tissue. While it reduces pain, it doesn’t fully restore hip motion or limb function and is generally reserved for smaller dogs or those for whom other surgeries are not feasible.
- Total Hip Replacement (THR): Considered the gold standard for severe, advanced osteoarthritis, THR involves replacing the damaged joint with prosthetic components. It offers the best chance for preserving long-term limb functionality and alleviating pain, but it’s an expensive and complex procedure.
[internal_links]
Breeding for Healthier Hips: Leveraging Genetic Information
Given the limitations of diagnosis and treatment, prevention through responsible breeding practices based on hip dysplasia in dogs genetics remains the most effective long-term strategy. Veterinarians and breeders are at the forefront of this effort, aiming to reduce the incidence and severity of CHD in future generations.
Traditional Breeding Programs and Their Limitations
For decades, CHD control programs have relied heavily on radiographic screening of breeding stock. Dogs with “low hip scores” (indicating good hip quality based on DJD signs and hip congruence) were selected for breeding. Systems like OFA, FCI, and BVA/KC have been instrumental in this.
However, these phenotype-based selection programs have faced several challenges:
- Environmental Influence: As CHD is multifactorial, a dog with a radiographically “normal” phenotype might still carry genes for dysplasia if environmental factors masked its genetic predisposition. These “carriers” can transmit the undesired genes to their offspring.
- Subjectivity: The scoring systems, while standardized, can still involve a degree of subjective interpretation by scrutinizers.
- Voluntary Participation: Many screening schemes are voluntary, meaning breeders are more likely to submit radiographs of dogs with good hips, leading to biased databases and an underestimation of the true prevalence of CHD within a breed.
- Slow Progress: Studies have shown that even with strict application, selection based solely on individual phenotypic hip quality has resulted in relatively slow genetic improvement, with only modest reductions in CHD prevalence over several decades in some breeds.
These limitations underscore the need for more sophisticated genetic tools to identify the true genetic quality of a potential breeding dog, beyond what is visible on an X-ray.
The Power of Estimated Breeding Values (EBVs) and Genomic Breeding Values (GBVs)
To overcome the shortcomings of phenotype-based selection, advanced genetic parameters like Estimated Breeding Values (EBVs) and Genomic Breeding Values (GBVs) are gaining traction.
- Estimated Breeding Values (EBVs): Commonly used in livestock breeding for complex polygenic traits (like milk yield or growth rate), EBVs for CHD are derived from the hip quality of a dog’s relatives (parents, siblings, offspring). By incorporating information from an entire pedigree, EBVs provide a more accurate estimation of a dog’s genetic potential for hip health, independent of environmental influences on its individual phenotype. This allows for better monitoring of genetic trends within dog populations and more informed breeding decisions.
- Genomic Breeding Values (GBVs): With rapid advancements in high-throughput sequencing and genome-wide single nucleotide polymorphism (SNP) arrays, it’s now possible to use direct molecular genetic information to estimate GBVs. Genomic selection, successfully applied in livestock, identifies associations between specific genetic markers (SNPs) and CHD genes. By genotyping a dog for a set of informative SNPs, combined with phenotypic and pedigree data, a GBV can be calculated. This offers the promise of higher selection pressure and more significant genetic improvement per generation, as it directly assesses a dog’s genetic makeup rather than relying solely on observable traits or family history. In the near future, GBVs are expected to become the recommended method for improving hip quality in CHD control schemes.
[internal_links]
Advancements in Genetic Research: GWAS and Identifying Susceptibility Alleles
The scientific community has long accepted the genetic etiology of CHD, fueling intensive research into the specific genes involved. Early molecular studies, pioneered in the 1990s at Cornell University, began by searching for molecular genetic markers linked to quantitative trait loci (QTLs) responsible for different CHD phenotypes. QTLs are regions of the genome that contain genes associated with a particular quantitative trait.
Early Genetic Marker Studies
Researchers initially crossbred breeds with high and low susceptibility to CHD (e.g., Labrador Retrievers and Greyhounds) to optimize the linkage of genes to CHD traits. These studies successfully identified several chromosomes harboring putative QTLs for various CHD traits, including the Norberg angle (a measure of hip joint laxity) and acetabular osteophyte formation (a sign of DJD). While these studies confirmed the polygenic nature of CHD, QTL regions can contain hundreds of genes, making the identification of specific causative genes problematic.
Genome-Wide Association Studies (GWAS) and Candidate Genes
The advent of Genome-Wide Association Studies (GWAS) has revolutionized the search for genetic variants associated with complex diseases like CHD. GWAS systematically scan the entire genome of a large population for genetic markers (typically SNPs) that are more common in affected individuals than in unaffected ones. This approach is more effective than individual SNP analysis because it considers the combined effect of multiple SNPs.
Through GWAS, researchers have identified numerous SNPs significantly associated with CHD in various dog breeds:
- In a study involving eight different dog breeds, four SNPs were linked to CHD on canine chromosomes (CFA) 3, 11, and 30.
- In German Shepherd dogs, several studies pinpointed SNPs associated with CHD on CFA14, 37, 19, 24, 26, 34, 3, 9, and 33.
- For Labrador Retrievers, GWAS identified SNPs on CFA1 and 21, and further studies found 31 SNPs across CFA1, 5, 8, 15, 20, 25, and 32. These SNPs were often located within or near genes potentially involved in cartilage formation, extracellular matrix integrity, and chondrocyte differentiation.
These findings strongly confirm the complex genetic architecture of CHD, where many genes contribute small individual effects. This complexity underscores why finding a single “marker-assisted, accurate CHD diagnostic test” has been difficult. Instead, the immediate importance of these molecular discoveries lies in their potential use for genomic selection, where multiple markers are assessed for their combined contribution to a dog’s hip health.
Comparing these advancements to human hip dysplasia research reveals a similar complexity. Genetic studies in humans have also struggled to make rapid progress, with understanding of loci-linked hip dysplasia still limited. The heterogeneity of human populations makes genetic dissection challenging, suggesting that insights from detailed canine studies can benefit analogous conditions in humans. [internal_links]
The Future of DNA Testing for Canine Hip Dysplasia
The dream of a simple DNA-based test for CHD that can accurately identify susceptible or resistant dogs early in life is a powerful motivator for both breeders and owners. Such a tool would allow for proactive management and more precise breeding decisions, significantly impacting the incidence of the disease.
Current Commercial DNA Tests
In 2012, Bioiberica registered “Dysgen,” the first commercial marker-based DNA test for susceptibility to CHD in Labrador Retrievers. This test analyzes blood samples using a DNA kit that targets seven specific SNPs. The results classify dogs into risk groups: minimal, low, moderate, or high for developing CHD. While a significant first step, the performance of the Dysgen test has not been independently validated through published studies, and its success in controlling CHD at a population level is still awaiting evidence. Manufacturers typically recommend breeding dogs with minimal or low risk, which aligns with responsible breeding practices.
Integrating Genetic Information for Comprehensive Control
The current reality is that until all contributing and critical mutations for CHD are identified across all affected breeds, no single DNA test will offer a complete solution. The genetic architecture of CHD is too complex for a simple “pass/fail” test. Therefore, the most effective CHD control programs will require a multi-pronged approach:
- Accurate Phenotype Screening: Continued use of established radiographic methods like PennHIP and OFA to assess the physical condition of the hips.
- Estimated Breeding Values (EBVs): Incorporating pedigree and phenotypic data to estimate a dog’s true genetic quality.
- Available Genetic Tests: Utilizing commercial DNA tests, like Dysgen, as an additional piece of the puzzle, especially for breeds where specific markers have been identified and validated.
- Genomic Breeding Values (GBVs): The integration of genomic data for a more precise estimation of genetic merit, allowing for higher selection pressure in breeding programs.
This comprehensive approach acknowledges the complexity of hip dysplasia in dogs genetics and combines all available tools to make the most informed breeding and management decisions. It’s particularly important when the heritability of a trait is moderate to low, as combining information from major gene genotypes and EBVs significantly enhances the effectiveness of selection. Considering the wide range of breeds affected and the varied environments they live in, a deep, integrated understanding of CHD genetics at the population level is essential for future success. [internal_links]
Conclusion
Canine Hip Dysplasia remains one of the most common and challenging orthopedic hereditary diseases in dogs, despite extensive phenotypic screening and breeding programs over the last 50 years. There is no ideal diagnosis or treatment, making reproductive control schemes a priority area in veterinary medicine.
The genetic architecture of CHD is undeniably complex, characterized by numerous associated genes each contributing a small individual effect. This inherent complexity has made the development of a single, highly accurate marker-assisted CHD diagnostic test a difficult endeavor, despite intensive research efforts worldwide.
Looking ahead, the molecular diagnosis and control of CHD will likely be based on genomic selection, integrating comprehensive genetic data until all critical contributing mutations are fully identified. This ongoing research into hip dysplasia in dogs genetics promises not only to revolutionize canine health but also to offer significant insights for a better understanding of similar human conditions, particularly developmental hip dysplasia and osteoarthritis. By combining responsible breeding practices with a thorough understanding of genetics and ongoing scientific advancements, we can work towards a future with healthier hips for our beloved canine companions.
Based on scientific literature and veterinary guidelines.
