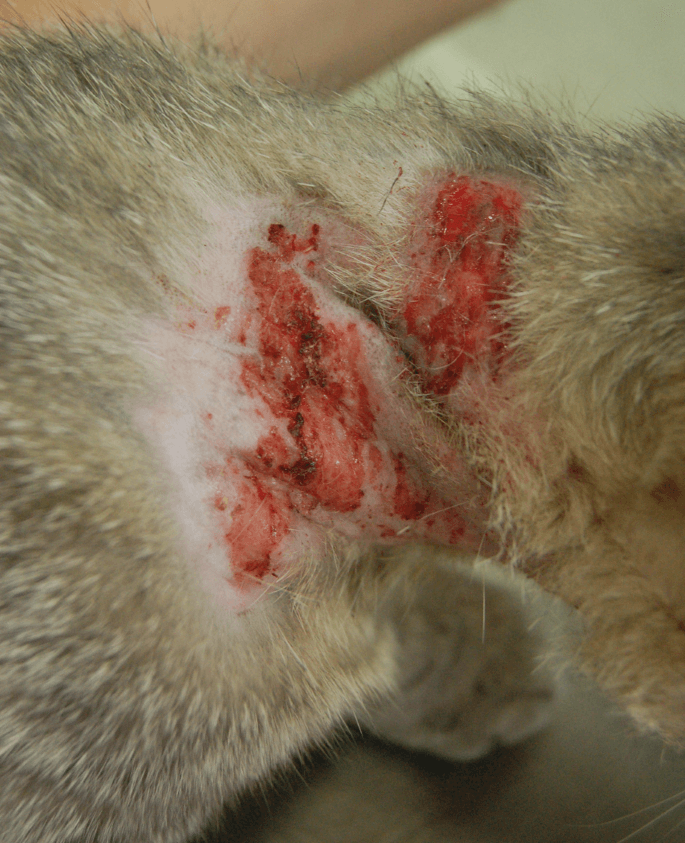
Feline idiopathic head-and-neck dermatitis (IHD), also known as feline idiopathic ulcerative dermatitis (IUD), is a rare skin condition of unknown origin. It typically manifests as a crusted, non-healing, self-inflicted ulcer, most commonly found on the dorsal or lateral neck or between the shoulder blades, areas cats frequently groom. IUD is usually diagnosed only after other causes of itching have been ruled out. In feline medicine, self-induced alopecia is recognized as a behavioral disorder, characterized by excessive licking that amplifies normal grooming behavior. These repetitive behaviors, including self-induced alopecia or wounds, are termed stereotypies and are considered indicators of poor welfare.
This study aimed to investigate whether the repetitive behavior associated with self-induced wounds in cats is linked to poor welfare and if improving the cat’s environment and their relationship with humans could lead to healing through environmental enrichment. Researchers recruited 13 cats diagnosed with IUD and assessed their welfare using a new 21-point welfare scale. The median welfare score for these cats was 16, significantly higher than the median score of 7 for 35 healthy cats, indicating a substantial difference in welfare. Major modifications to the cats’ environments and their human-cat relationships were recommended. Within 15 days of these environmental changes, the ulcerative lesions healed, and welfare scores improved significantly, reaching a median of 6, comparable to healthy cats. Only one cat, whose owners were reluctant to implement environmental changes and received psychotropic medication instead, did not heal. These findings suggest that feline IUD is a behavioral disorder indicative of poor welfare, requiring management by behavior specialists with a focus on environmental modifications. Based on these results, the authors propose renaming the condition “behavioral ulcerative dermatitis.”
Understanding Feline Head and Neck Pruritus
In cats, head and neck pruritus (HNP) is a dermatological syndrome characterized by itching in the head and/or neck region, accompanied by skin lesions. These lesions are often self-induced erosions or ulcerations that can exacerbate primary inflammatory skin conditions like miliary dermatitis, eosinophilic plaques, or urticarial papules. HNP can be triggered by an itchy sensation without an underlying inflammatory skin condition or can be a secondary symptom of a pruritic dermatosis. While multiple causes exist for HNP, some cases remain unidentified, leading to a diagnosis of feline idiopathic HNP, or IUD.
IUD was first reported in 1990 and is considered a rare skin disease of unknown origin. It is characterized by self-inflicted lesions, typically around the neck, temporal areas, or between the scapulae. Clinically, these lesions are erosive or ulcerative, sometimes with deep ulcers surrounded by thickened skin. They can be symmetrical or asymmetrical. Peripheral lymphadenomegaly may be present due to inflammation or secondary infection, but cats with IUD typically show no signs of systemic illness.
Differential diagnoses for IUD are broad and include foreign body reactions, trauma, thermal burns, erythema multiforme, bacterial, fungal, or viral infections, parasitic infestations, hypersensitivity disorders, neuropathic disorders, and neoplasia. Histopathological examination usually reveals extensive epidermal ulceration and superficial dermal necrosis with mild to moderate dermal inflammation. Chronic lesions may also show subepidermal fibrosis.
Challenges in Treating IUD
IUD lesions typically heal spontaneously once self-mutilation is prevented through measures like an Elizabethan collar or bandaging. However, the condition often proves refractory to most medications, with corticosteroids providing only short-term relief before relapse. Anecdotal successful treatments have been reported with medications like topiramate, gabapentin, cyclosporine, or oclacitinib. Surgical excision is sometimes attempted but often unsuccessful. Consequently, the prognosis for IUD is guarded, with a high risk of relapse after treatment cessation. The underlying cause remains poorly understood, making effective, long-term treatment a challenge.
The Link Between Grooming Disorders and Welfare
In contrast, other feline grooming disorders, such as self-induced alopecia, are increasingly recognized as psychogenic and are referred to as “stress-related overgrooming.” Environmental stress has been identified as a contributing factor, and treatments often involve environmental modifications and antidepressant medications. In various animal species, abnormal repetitive behaviors (ARBs), or stereotypies, are considered indicators of welfare problems. Overgrooming in cats, which dedicate a significant portion of their time budget to grooming, is particularly noted as a sign of poor welfare. Grooming, encompassing licking, biting, and scratching, represents approximately 4% of a cat’s daily activity. Scratching, specifically, is concentrated on the neck and head and can increase dramatically if a cat is prevented from scratching for a period. The control of grooming behavior is believed to be centrally regulated in cats.
Defining and Assessing Animal Welfare
Animal welfare is a complex concept encompassing complete mental and physical health, harmony with the environment, positive emotions, and successful adaptation with minimal stress. Established frameworks, like the Welfare Quality® and AWIN (Animal Welfare INdicators) protocols, assess welfare through principles such as good feeding, good housing, appropriate behavior, and good health, using specific criteria. For instance, the presence of stereotypies is considered an indicator of poor welfare in horses. However, similar comprehensive welfare scores have not been widely established for dogs and cats.
Study Design and Methodology
Study Design
This prospective, open-controlled study aimed to investigate the welfare status of cats with IUD. The study was conducted at Alfort School Veterinary Hospital (CHUVA) between January 2014 and January 2016. All cats involved were patients of the hospital, and their owners provided written informed consent. Ethical approval was obtained from the CHUVA ethics committee.
Welfare Score Development
A novel welfare score was developed for cats, drawing upon principles from existing welfare assessment protocols for cattle, pigs, poultry, and horses (Welfare Quality® and AWIN). This score aimed to evaluate the cat’s control over its resources and environment. Owners completed a detailed questionnaire covering aspects such as:
- Access to Resources: Free access to food, water, litter boxes, hiding spots, and resting areas.
- Owner Control: Whether resources were accessed by the cat freely, upon request, or entirely controlled by the owner.
- Human-Cat Relationship: The nature of interactions, whether initiated by the owner, the cat, or mutually.
- Inter-Cat Relationship: If multiple cats were present, the dynamics between them.
- Environmental Enrichment: The presence of objects for play and exploration.
- Environmental Adequacy: The fit between the cat’s genetic and individual needs and its living environment, based on Fraser et al.’s conceptual model.
The scoring system used a numeric scale where answers corresponding to better welfare received lower scores (closer to 0), and answers indicating poorer welfare received higher scores (up to 2). The total score ranged from 0 (absence of welfare problems) to 21 (serious welfare problems).
Animal Cohorts
Healthy Cats (Control Group)
Thirty-five healthy cats were recruited from the preventive veterinary medicine service during routine vaccination appointments. Cats younger than six months, or those with chronic diseases, dermatological conditions, or known behavioral disorders, were excluded. Owners completed the welfare questionnaire, and each cat received a global welfare score.
IUD Cats (Patient Group)
Thirteen cats diagnosed with IUD by a dermatology specialist (ECVD diplomate) were recruited from the dermatology service. The diagnosis was based on characteristic clinical signs: self-induced lesions or excoriations on the head and neck, consistent with normal grooming areas, and the exclusion of other pruritic dermatoses. Differential diagnoses considered and ruled out included atopic dermatitis, adverse food reactions, flea allergy dermatitis, parasitic infestations, bacterial/fungal dermatitis, metabolic conditions, and other inflammatory dermatoses. All IUD cats had received regular flea control for at least three months prior to the study. Concomitant medication use was not an exclusion criterion. After the dermatological assessment, cats were referred to a behavioral specialist for welfare evaluation using the newly developed score. A baseline welfare score (S1) was recorded during the initial consultation, followed by a second score (S2) during a recheck appointment, typically 15 to 90 days later.
Behavioral Treatment: Environmental Enrichment
The behavioral consultation involved a comprehensive assessment of the cat’s environment, including its genetic background, developmental history, temperament, access to resources, time budget, and relationships with humans and other animals. The goal was to identify potential stressors and discrepancies between the cat’s ethological needs and its living conditions. Based on the hypothesis that IUD stems from such environmental mismatches, the treatment focused on environmental modifications designed to improve welfare and reduce abnormal behaviors. Recommendations, tailored to each cat, included:
- Removing Frustrations and Restoring Control: Ensuring permanent access to food (using low-calorie options and foraging toys for overeaters) and water (offering water fountains if cats preferred running water). Providing opportunities for exploration and exercise, such as secure access to balconies or gardens, and recommending cat flap installation.
- Improving the Cat-Human Relationship: Advising owners to cease initiating interactions and instead allow the cat to initiate them, reinforcing positive interactions with treats.
- Managing Inter-Cat Relationships: In multi-cat households, ensuring each cat had access to secure, undisturbed areas for eating, sleeping, and elimination. In some cases, temporary spatial separation was advised.
- Adapting Time-Budget and Environment: Recommending regular introduction of new toys and providing high and hidden resting areas. Ensuring cats could use spaces like open closets as hiding spots if desired.
Medical Treatment
During the study period, no specific medical treatments were prescribed for the IUD cats, with environmental changes being the sole intervention. Any medications prescribed before the behavioral consultation were discontinued. The only exception was cat 13, whose owners could not implement environmental modifications. This cat received fluoxetine (1 mg/kg daily for one month) followed by imepitoin (10 mg/kg twice daily for another month) with no reported improvement.
Statistical Analysis
Welfare scores between healthy cats and IUD cats at inclusion (S1) were compared using the Mann-Whitney U test due to the non-parametric distribution of the data. For comparing the welfare scores of IUD cats before (S1) and after (S2) behavioral treatment, the Wilcoxon signed-rank test was employed. A significance level of p < 0.05 was used for all statistical tests.
Study Findings
Animal Demographics
Healthy Cats: The control group comprised 35 cats, primarily Domestic Shorthairs, with one Chartreux and one Blue Russian. Their ages ranged from 1 to 16 years (median: 7 years). Fifteen were castrated males and 20 were neutered females. Housing varied, with 6 cats having outdoor access, 19 having controlled access, and 10 being strictly indoor cats.
IUD Cats: Thirteen cats were diagnosed with IUD. With the exception of cat 2, all were referred by general veterinarians due to treatment failures. The group consisted of 6 males (5 castrated) and 7 neutered females. Ages ranged from 10 months to 8.5 years (median: 31 months). Breeds included Domestic Shorthair (7 cats), Maine Coon (3 cats), British Shorthair (1 cat), and Scottish Fold (1 cat). Eleven cats lived exclusively indoors, while two had both indoor and outdoor access but could be confined indoors. Four cats had histopathological findings consistent with IUD. Eleven cats had previously received corticotherapy, with 9 owners reporting temporary improvement. Two cats (cases 12 and 13) showed no improvement with previous treatments.
Dermatological Presentation
The onset of IUD varied, with the median age at onset being 19 months (range: 6–40 months). The duration of the disease before presentation ranged from 1 month to 8 years (median: 9 months). The primary clinical sign in all IUD cats was excoriations.
Lesion localization varied but consistently involved the head (4 cats), shoulder (1 cat), and/or neck (10 cats). Two cats had head lesions only (retroauricular or temporal areas). The majority (10 cats) had cervical lesions, with two of these also presenting lesions on the chin or temporal area. Eight cats had a single localized lesion, while one had two, and four had multiple lesions.
Welfare Scores and Treatment Outcomes
Welfare Score Comparison: Healthy cats exhibited significantly lower welfare scores (indicating better welfare) at inclusion compared to IUD cats (median score: 7 vs. 16, p < 0.001). This stark difference underscores the compromised welfare state in IUD-affected cats.
Post-Treatment Improvement: Following the implementation of environmental modifications, the welfare scores of IUD cats significantly decreased (median score: 6, p < 0.001), reaching levels comparable to those of healthy cats (no significant difference). This dramatic improvement in welfare scores occurred concurrently with clinical recovery.
Clinical Healing: Pruritus ceased within two days of initiating environmental changes in all cats. Skin lesions healed rapidly in the subsequent days, with or without scarring depending on the depth of the wounds. All 13 cats healed, except for cat 13, whose owners did not implement the recommended environmental changes. This cat remained untreated by behavioral means and did not heal, necessitating psychotropic medication without success.
Relapse and Follow-up: For 12 out of the 13 cats (92%), clinical signs did not relapse during a follow-up period ranging from 12 to 24 months. This high rate of sustained healing highlights the efficacy of the environmental intervention.
Discussion
This study presents compelling evidence that Feline Idiopathic Ulcerative Dermatitis (IUD) is not merely a dermatological condition but rather a manifestation of underlying behavioral issues linked to poor welfare. Similar to how psychogenic pruritus in humans can be misdiagnosed as idiopathic due to a lack of diagnostic tools, IUD may have been similarly miscategorized in cats. This misdiagnosis can have significant implications for both the cat’s quality of life and the owner-cat relationship, potentially leading to difficult decisions such as euthanasia.
IUD as a Manifestation of Abnormal Repetitive Behaviors and Poor Welfare
The central regulation of grooming behavior in cats, as previously established, suggests that disruptions to this control can lead to abnormal repetitive behaviors like fur chewing, which are observed in other species under poor welfare conditions. The known impact of environmental stress on conditions like idiopathic cystitis in cats further supports the link between stress, environment, and disease. The concept of “Pandora syndrome,” which describes chronic conditions in cats with comorbid behavioral, dermatological, and other disorders, further suggests a systemic link between stress and various ailments. This study extends this concept by demonstrating a measurable link between poor welfare and IUD.
The development of a specific cat welfare score, based on established principles, allowed for a quantitative assessment of welfare in IUD cats. The significantly higher welfare scores in IUD cats compared to healthy controls provide strong evidence that these cats are experiencing compromised welfare. Crucially, the improvement in both welfare scores and clinical condition following environmental modifications underscores the effectiveness of addressing the underlying welfare issues.
The rapid healing observed across nearly all cats after implementing environmental changes suggests that these modifications directly address the root cause of the condition. While the study did not isolate the impact of individual environmental changes due to their simultaneous implementation, the observation that free access to the outdoors, for example, contributed significantly to welfare score improvement (a 10-point drop was noted) suggests that providing greater environmental control and stimulation is key.
Re-framing IUD: From “Idiopathic” to “Behavioral” Ulcerative Dermatitis
Given the findings, the authors propose renaming IUD to “behavioral ulcerative dermatitis” or “self-induced ulcerative dermatitis.” This reclassification reflects the understanding that the condition stems from behavioral disorders linked to environmental factors and poor welfare. The prognosis appears favorable when owners are able and willing to implement the recommended environmental and relational changes.
The study also touches upon the potential for genetic predisposition, noting that breed cats were overrepresented in the IUD group compared to the general cat population. This aligns with the concept that some individuals may be genetically more susceptible to environmental stress or mal-adaptation.
A Positive Diagnosis, Not Just by Exclusion
The authors advocate for viewing behavioral ulcerative dermatitis not as a diagnosis of exclusion, but as a positive diagnosis. This involves identifying both the absence of a somatic cause and the presence of characteristic clinical signs: self-induced lesions in grooming areas, and an association with a poor welfare score. While a chronological link to specific life events is often not reported by owners, the clinical presentation and welfare assessment are key diagnostic indicators.
The differential diagnosis must always include other dermatological diseases. However, the pattern of self-induced lesions and the specific locations (neck, temporal area, shoulder) are highly suggestive of a behavioral origin. While primary dermatological conditions can be exacerbated by self-trauma, the absence of primary inflammatory lesions or characteristic topographical distribution points away from a purely dermatological diagnosis.
Pathogenesis: A Complex Interaction
The pathogenesis of pruritus, in both humans and potentially cats, involves complex interactions between sensory, motor, and affective brain areas. Chronic skin inflammation from scratching can lead to peripheral and central sensitization, intensifying itch and potentially contributing to psychogenic components. The temporary effectiveness of treatments like corticosteroids might be explained by their anti-inflammatory action on both peripheral and central pathways. Furthermore, brain inflammation has been implicated in obsessive-compulsive disorders in humans, suggesting a potential parallel with behavioral ulcerative dermatitis in cats.
Conclusion
This research marks a significant step in understanding feline idiopathic ulcerative dermatitis (IUD). For the first time, IUD is considered a behavioral disease strongly indicative of poor welfare. The study proposes renaming it “behavioral ulcerative dermatitis” based on three key points:
- All cutaneous lesions are self-induced, resulting from scratching in grooming areas.
- This repetitive behavior is associated with a demonstrably poor welfare score.
- Complete healing occurs following environmental modifications that address the cat’s specific ethological needs.
Further research with larger cat populations is warranted to further define this behavioral disorder and its link to environmental adaptation. The developed welfare scoring system could also prove valuable in investigating other repetitive behaviors in cats, such as tail chasing or self-induced alopecia.
Ethics Statement
All cats involved in this study were patients of the CHUVA, and their treatment and participation were conducted in accordance with the hospital’s ethical guidelines. Owners provided written informed consent for their cats’ inclusion in the study.
Author Contributions
ET and NC-F conceptualized the study. AB, NC-F, and ET were involved in case recruitment. CG developed the welfare score. All authors contributed to drafting, reviewing, and approving the final manuscript.
Conflict of Interest Statement
The authors declare no commercial or financial relationships that could be construed as a potential conflict of interest.
References
- Scott DW. An unusual ulcerative dermatitis associated with linear subepidermal fibrosis in eight cats. Feline Pract (1990) 18:8–18.
- Spaterna A, Mechelli L, Rueca F, Cerquetella M, Brachelente C, Antognoni MT, et al. Feline idiopathic ulcerative dermatosis: three cases. Vet Res Commun (2003) 27:795–8. doi:10.1023/B:VERC.0000014274.32708.40
- Miller WH, Griffin CE, Campbell KL, editors. Miscellaneous skin diseases. 7th ed. Muller and Kirk’s Small Animal Dermatology. St Louis, MO: Elsevier (2013). p. 718–9.
- Rusbridge C, Heath S, Gunn-Moore DA, Knowler SP, Norman Johnston N, McFadyen AK. Feline orofacial pain syndrome (FOPS): a retrospective study of 113 cases. J Feline Med Surg (2010) 12:498–508. doi:10.1016/j.jfms.2010.03.005
- Hnilica KA, editor. Hypersensitivity disorders. 3rd ed. Small Animal Dermatology: A Color Atlas and Therapeutic Guide. St Louis, MO: Elsevier (2011). p. 219–20.
- Grant D, Rusbridge C. Topiramate in the management of feline idiopathic ulcerative dermatitis in a two-year-old cat. Vet Dermatol (2014) 25:226–8, e59–60. doi:10.1111/vde.12124
- Gross TL, Ihrke PJ, Walder EJ, Affolter VK, editors. Feline idiopathic ulcerative dermatosis. 2nd ed. Skin Diseases of the Dog and Cat: Clinical and Histopathological Diagnosis. Oxford: Blackwell Science (2005). p. 130–2.
- Loft KE. Feline idiopathic ulcerative dermatosis treated successfully with oclacitinib. Vet Dermatol (2015) 26:134–5. doi:10.1111/vde.12213
- Sawyer LS, Moon Fanelli AA, Dodman NH. Psychogenic alopecia: 11 cases (1993–1996). J Am Vet Med Assoc (1999) 214:71–4.
- Overall KL. Manual of Clinical Behavioural Medicine for Dogs and Cats. St Louis, MO: Elsevier (2013). p. 442–3.
- Bowen J, Heath S, editors. Feline compulsive disorders. Behaviour Problems in Small Animals: Practical Advice for the Veterinarian Team. Philadelphia, PA: Elsevier Saunders (2005). p. 177–81.
- McAfee LM, Mills DS, Cooper JJ. The use of mirror for the control of stereotypic weaving behaviour in the stabled horse. Appl Anim Behav Sci (2002) 78:159–73. doi:10.1016/S0168-1591(02)00086-2
- Würbel H. The motivational basis of caged rodents stereotypies. 2nd ed. In: Mason G, Rushen J, editors. Stereotypic Animal Behaviour. Fundaments and Applications to Welfare. London, UK: CABI (2006). p. 86–120.
- Seksel K, Lindeman M. Use of clomipramine in the treatment of anxiety-related and obsessive-compulsive disorders in cats. Aust Vet J (1998) 76:317–21. doi:10.1111/j.1751-0813.1998.tb12353.x
- Luescher AU. Diagnosis and management of compulsive disorders in dogs and cats. Clin Tech Small Anim Pract (2004) 19:233–9. doi:10.1053/j.ctsap.2004.10.005
- Mason GJ, Latham NR. Won’t stop, can’t stop: is stereotypy a reliable animal welfare indicator? Anim Welf (2004) 13:57–69.
- Broom DM. Stereotypies as animal welfare indicators. In: Schmidt D, editor. Indicators Relevant to Farm Animal Welfare. The Hague, The Netherlands: Martinus Nijhoff (1983). p. 81–7.
- Parker M, Redhead ES, Goodwin D, McBride SD. Impaired instrumental choice in cribbing horses (Equus caballus). Behav Brain Res (2008) 191:137–40. doi:10.1016/j.bbr.2008.03.009
- Franchi V, Aleuy OA, Tadich TA. Fur chewing and other abnormal repetitive behaviors in chinchillas (Chinchilla lanigera), under commercial fur-farming conditions. J Vet Behav (2016) 11:60–4. doi:10.1016/j.jveb.2015.10.002
- Eckstein RA, Hart BL. The organization and control of grooming in cats. Appl Anim Behav Sci (2000) 68:131–40. doi:10.1016/S0168-1591(00)00095-2
- Beaver BV. Feline Behaviour: A Guide for Veterinarians. 2nd ed. St Louis, Missouri: Elsevier (2002). p. 311–3.
- Hughes BO. Preference decisions of domestic hens for wire or litter floors. Appl Anim Ethol (1976) 2:155–65. doi:10.1016/0304-3762(76)90043-2
- Broom DM. Indicators of poor welfare. Br Vet J (1986) 142:524–6. doi:10.1016/0007-1935(86)90109-0
- Fraser D, Weary DM, Pajor EA, Milligan BN. A scientific conception of animal welfare that reflects ethical concerns. Anim Welf (1997) 6:187–205.
- Botreau R, Veissier I, Perny P. Overall assessment of animal welfare: strategy adopted on welfare quality®. Anim Welf (2009) 18:363–70.
- Awin. Awin Welfare Assessment Protocol for Horses (2015). Available from: https://air.unimi.it/retrieve/handle/2434/269097/384836/AWINProtocolHorses.pdf (Accessed: February 4, 2018).
- Welfare Quality®. Welfare Quality® Assessment Protocol for Dairy Cows. Lelystad: Welfare Quality® Consortium (2009).
- Ellis S. Environmental enrichment: practical strategies for improving welfare. J Feline Med Surg (2009) 11:901–12. doi:10.1016/j.jfms.2009.09.011
- Herron ME, Buffington CAT. Environmental enrichment for indoor cats. Compend Contin Educ Vet (2010) 32:E1–5.
- Heath S, Wilson C. Canine and feline enrichment in the home and kennel. Vet Clin North Am Small Anim Pract (2014) 44:427–49. doi:10.1016/j.cvsm.2014.01.003
- Ellis S, Rodan I, Carney H, Heath S. AAFP and ASFM feline environmental needs guidelines. J Feline Med Surg (2013) 15:219–30. doi:10.177/1098612X13477537
- Misery L. Psychogenic pruritus. In: Misery L, Ständer S, editors. Pruritus. London: Springer-Verlag (2016). p. 307–12.
- Westropp JL, Kass PH, Buffington CAT. Evaluation of the effect of stress in cats with idiopathic cystitis. Am J Vet Res (2006) 67:731–6. doi:10.2460/ajvr.67.4.731
- Buffington CAT, Westropp JL, Chew DJ, Bolus RR. Clinical evaluation of multimodal environmental modification (MEMO) in the management of cats with idiopathic cystitis. J Feline Med Surg (2006) 8:261–8. doi:10.1016/j.jfms.2006.02.002
- Buffington CAT, Westropp JL, Chew DJ. From FUS to Pandora syndrome. Where are we, how did we get here, and where to now? J Feline Med Surg (2014) 16:385–95. doi:10.1177/1098612X14530212
- Overall KL, Dunham AE. Clinical features and outcomes with in dogs and cats with obsessive-compulsive disorder: 126 cases (1989–2000). J Am Vet Med Assoc (2002) 221:1445–52. doi:10.2460/javma.2002.221.1445
- Luescher A. Compulsive behavior in companion animals. Recent advances in companion animal behavior problems. In: Houpt KA, editor. International Veterinary Information Service. Ithaca, NY (2000). Available from: www.ivis.org (Accessed: September 22, 2000).
- Tynes VV, Sinn L. Abnormal repetitive behaviors in dogs and cats: a guide practitioners. Vet Clin North Am Small Anim Pract (2014) 44:43–564. doi:10.1016/j.cvsm.2014.01.011
- La revue de l’alimentation animale. (2010). Available from: http://www.revue-alimentation-animale.fr/petfood-nutrition-formulation/enquete-faccotns-sofres-60-millions-danimaux-familiers-en-france/ (Accessed: June 8, 2010).
- Low M. Stereotypies and behavioural medicine: confusions in current thinking. Aust Vet J (2003) 81:192–8. doi:10.1111/j.1751-0813.2003.tb11468.x
- Héripret D, Carlotti DN. Dermatite atopique féline: données actuelles. Pratique Med Chir Anim Comp (2010) 45:79–87. doi:10.1016/j.anicom.2010.07.003
- Gieler U, Niemeier V, Brosig B, Kupfer J. Psychosomatic aspect of pruritus. Dermatol Psychosom (2002) 3:6–13. doi:10.1159/000051357
- Darsow U, Drzezga A, Frisch M, Munz F, Weilke F, Bartenstein P, et al. Processing of histamine-induced itch: a correlation analysis with dermal reactions. J Invest Dermatol (2000) 115:1029–33. doi:10.1046/j.1523-1747.2000.00193.x
- Drzezga A, Darsow U, Treede RD, Siebner H, Frisch M, Munz F, et al. Analogies to pain processing: a correlational analysis of O-15 H2O positron emission tomography studies. Pain (2001) 92:295–305. doi:10.1016/S0304-3959(01)00271-8
- Mochizuki H, Tashiro M, Kano M, Sakurada Y, Itoh M, Yanai K. Imaging of central itch modulation in the human brain using positron emission tomography. Pain (2003) 105:339–46. doi:10.1016/S0304-3959(03)00249-5
- Walter B, Sadlo MN, Kupfer J, Niemeier V, Brosig B, Stark R, et al. Brain activation by histamine prick test-induced itch. J Invest Dermatol (2005) 125:380–2. doi:10.1111/j.0022-202X.2005.23817.x
- Paus R, Schmelz M, Biro T, Steinhoff M. Frontiers in pruritus research: scratching the brain for more effective itch therapy. J Clin Invest (2006) 116:1174–85. doi:10.1172/JCI28553
- Attwells S, Setiawan E, Wilson AA, Rusjan PM, Mizrahi R, Miller L, et al. Inflammation in neurocircuitry of obsessive-compulsive disorder. JAMA Psychiatry (2017) 74:833–40. doi:10.1001/jamapsychiatry.2017.1567