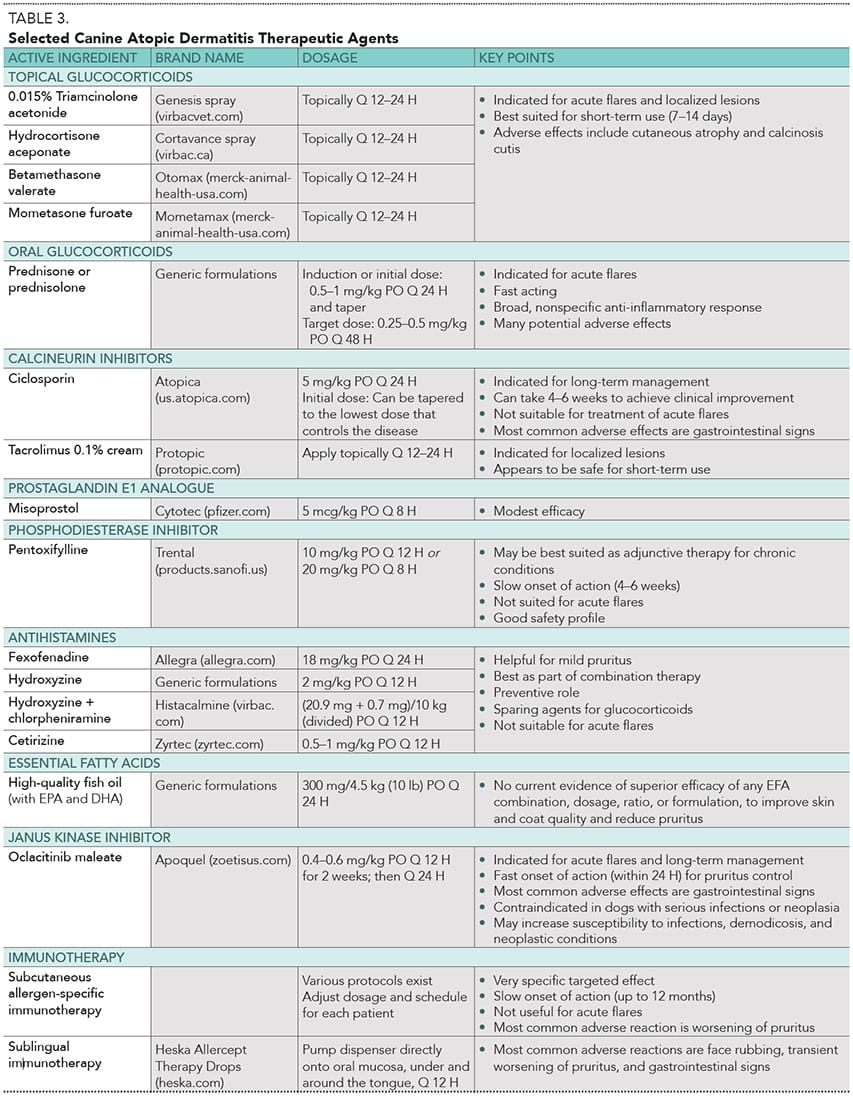
Apoquel®, with the generic name oclacitinib, is a significant advancement in veterinary dermatology, offering a novel approach to managing pruritus and inflammatory skin conditions in pets. As a Janus Kinase (JAK) inhibitor, it targets specific signaling pathways involved in itch and inflammation, differentiating it from traditional treatments like steroids and antihistamines. This article delves into clinical experiences and considerations surrounding Apoquel’s use, aiming to provide a comprehensive understanding for pet owners and veterinary professionals alike.
Understanding Apoquel’s Mechanism and Administration
Apoquel functions by inhibiting JAK-1 and JAK-3 signaling. This mechanism effectively blocks the action of cytokines, such as IL-31, which are primary mediators of itch. Its rapid onset of action is a key benefit, with many dogs experiencing noticeable relief from itching within 24 hours of the initial dose. Unlike some other immunosuppressants, Apoquel generally has a low incidence of gastrointestinal upset. However, its antipruritic effects are relatively short-lived, necessitating daily administration for sustained control in most cases.
Dosing and Administration for Dogs
The prescribed dosage for dogs is typically 0.4-0.6 mg/kg every 12 hours for the first 14 days, followed by a maintenance dose of 0.4-0.6 mg/kg once daily. It’s crucial to adhere to these guidelines, as exceeding the recommended dose can increase the risk of immune suppression, while doses below the range may prove ineffective for many dogs. While Apoquel is FDA-approved for twice-daily dosing for up to 14 days, prolonged use at this frequency is considered off-label. Zoetis reports that approximately 60% of dogs with moderate to severe atopic dermatitis achieve long-term control with once-daily dosing, though some severe cases may benefit from extended twice-daily use under veterinary supervision. Often, managing severe skin disease requires a multimodal approach combining Apoquel with other therapies.
Potential Drug Interactions and Combination Therapies
While specific long-term studies on combining Apoquel with other immunosuppressants like steroids and cyclosporine are limited, clinical experience suggests that short-term concurrent use with anti-inflammatory doses of steroids (e.g., 0.5mg/kg twice daily, with a taper) can be safe. It is important to assess the efficacy of Apoquel when steroids are needed, as its rapid action means a slow transition from steroids is often unnecessary. However, tapering steroids might still be required to prevent Addisonian crisis in pets previously on high-dose or daily steroid therapy for extended periods. Caution is advised when administering Apoquel with other drugs that might affect bone marrow function.
Age Restrictions and Efficacy in Young Puppies
Apoquel is FDA-approved for dogs over 12 months of age. This restriction stems from studies where higher doses of Apoquel (3x and 5x the regular dose) in dogs under one year old led to an unacceptable incidence of demodicosis and pneumonia. Clinically, Apoquel often demonstrates limited efficacy for allergic conditions in very young puppies.
Side Effects and Clinical Observations in Dogs
While Apoquel is generally well-tolerated, potential side effects, as listed in the product insert, include vomiting, diarrhea, lethargy, anorexia, skin masses, decreased leukocytes, decreased globulins, and increased cholesterol and lipase. Less common, but reported, side effects include demodicosis, neoplasia, pneumonia, bloody diarrhea, skin and ear infections, UTIs, and histiocytomas. Some studies noted transient side effects like polydipsia, increased appetite, and aggression, which can be similar to steroid-induced effects, although a direct causal link to Apoquel has not been definitively established in all cases.
Dr. Eisenschenk’s Clinical Experience
With extensive experience starting over 1000 dogs on Apoquel, Dr. Eisenschenk notes that adverse effects are rare. Bone marrow suppression, observed in approximately 1% of patients, is the most significant concern. This is typically detected through routine bloodwork, often without overt clinical signs. Regular blood monitoring (CBC/Chem6) is recommended at 3 months and then annually to detect such changes. Decreasing the Apoquel dosage usually allows the bone marrow to recover. While other atopic dermatitis medications do not typically cause bone marrow suppression, allergy testing and immunotherapy remain the only non-immunosuppressive treatment options.
Ear infections are often managed more effectively with steroids or cyclosporine than with Apoquel. Urinary tract infections have also been observed, though their direct relation to Apoquel versus the underlying atopic dermatitis is debated. Weight gain, similar to that seen with JAK inhibitors in humans, can occur but is generally less pronounced than with steroids. Rare instances of behavioral changes, described as manic episodes, have been reported, resolving upon discontinuation of Apoquel. Intriguingly, some dogs have shown improved mobility, potentially due to reduced overall inflammation.
Regarding skin masses, histiocytomas are more common in dogs on Apoquel and typically resolve spontaneously. Viral papillomas may also be exacerbated but often improve when Apoquel is stopped. The concern that Apoquel may exacerbate neoplastic conditions is noted, but robust evidence is lacking. Many reports linking Apoquel to cancer are likely instances where underlying internal diseases, including cancer, triggered the severe skin issues for which Apoquel was prescribed.
Monitoring Protocols for Apoquel Use
The recommended monitoring protocol for dogs on once-daily Apoquel involves a CBC/Chem6 prior to initiation, followed by checks at 3 months and then annually. The CBC is paramount for detecting potential bone marrow suppression. Pre-existing liver disease should be ruled out before starting Apoquel, with the exception of liver elevations attributed to steroid use.
Summary of Apoquel’s Benefits and Limitations
Apoquel is favored for its rapid onset of action, low incidence of GI side effects, and rare occurrence of serious adverse events with long-term use. However, the need for bone marrow monitoring, its ineffectiveness in some pets, and the limited long-term data are notable drawbacks. Despite these considerations, it remains a valuable therapeutic option for many dogs suffering from atopic dermatitis.
It is essential to read the drug insert for detailed information and FDA-recommended usage. Adverse side effects should be reported to the FDA or the manufacturer, Zoetis. For specific medical advice regarding Apoquel, consulting a veterinarian is paramount, as the clinic cannot provide medical guidance for patients not seen in person.
Apoquel Use in Cats: Off-Label Considerations
While Apoquel is not FDA-approved for cats, it can be used to manage atopic dermatitis and other immune-mediated diseases. Cats typically require higher doses and more frequent administration than dogs. While some feline patients respond well, others do not. Monitoring bloodwork is crucial, with checks at 2, 5 months, and then every six months. In one review of 166 cats, 66% showed good control, but 7% discontinued treatment due to low immune cell counts (neutropenia) within 2-5 months. Apoquel was ineffective for 34% of cats, with some experiencing vomiting, diarrhea, or lethargy, or simply not showing improvement after a trial period. Due to the off-label nature and potential side effects, management of feline cases requiring Apoquel is best handled by a veterinary dermatologist.