Introduction
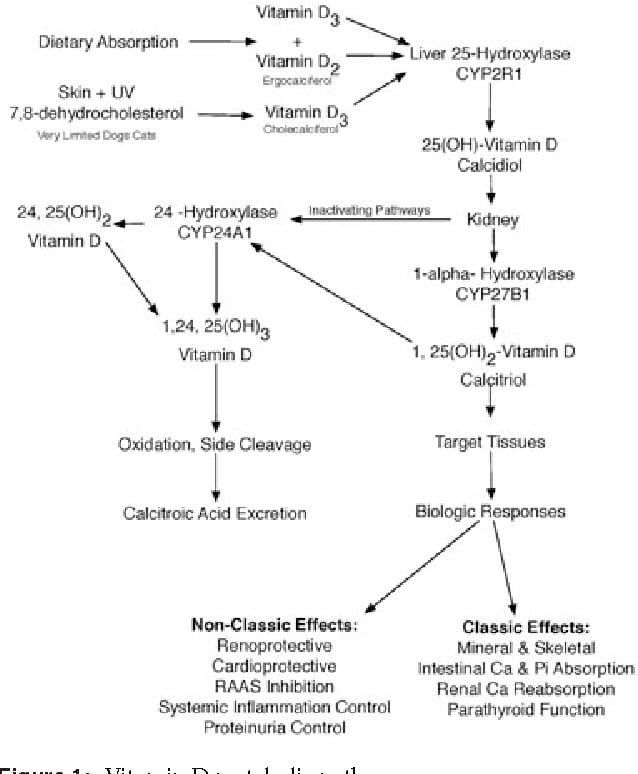
Calcium (Ca) homeostasis is a complex process in cats, intricately regulated by hormones such as calcitriol, parathyroid hormone (PTH), and calcitonin. These hormones orchestrate the mobilization of calcium and phosphate from bones, influence renal reabsorption, and modulate intestinal absorption. Fibroblast growth factor 23 (FGF23) also plays a role, primarily in phosphorus metabolism, by increasing renal phosphorus excretion and decreasing plasma calcitriol concentrations. This study delves into the physiological impact of varying dietary calcium concentrations on calcium homeostasis and vitamin D metabolism in healthy adult cats, building upon previous research that suggested a dietary regulation of calciotropic hormones. The study aims to provide new insights into these regulatory mechanisms, particularly concerning the interaction between dietary calcium and vitamin D metabolites.
Materials and Methods
Experimental Design and Animal Welfare
The study involved ten healthy adult cats, with diets formulated to meet the recommendations for adult cats. Six experimental diets with increasing concentrations of dicalcium phosphate were administered over six feeding periods. Each period included a 10-day adaptation phase followed by an 8-day sampling phase for urine and feces. Blood samples were collected on the final day of each feeding period. The experimental protocol was approved by the relevant Animal Welfare Committee. To mitigate potential carry-over effects, diets were fed in ascending order of calcium concentration.
Animal Housing and Sampling
Cats were housed individually in metabolic cages during the sampling periods under controlled environmental conditions. They were fed twice daily, and water was available ad libitum. Body weight was monitored weekly. Urine was collected twice daily, with pH measured before storage at -40°C. Fecal samples were also collected twice daily, and after manual removal of litter pellets, were lyophilized and stored at -40°C for analysis. Blood samples were drawn after an 18-hour fast.
Diets and Nutrient Analysis
Six experimental diets were formulated with varying calcium and phosphorus levels, ranging from 6.23/6.02 to 24.3/21.6 g/kg dry matter (DM). Dicalcium phosphate served as the calcium source. A standard vitamin and trace element premix was used across all diets, with vitamin D3 included. Nutrient analysis of the diets was conducted according to established methods. The base excess (BE) was calculated to predict urinary pH.
Blood and Urine Analysis
Blood samples were analyzed for urea, creatinine, mineral concentrations, PTH, FGF23, and vitamin D metabolites (25(OH)D2 and 25(OH)D3) using validated methods. Urinary pH was measured using an electronic pH meter. Urine samples were acidified, filtered, and analyzed for mineral and oxalate concentrations.
Fecal Sample Preparation
Fecal samples were lyophilized, ground to a fine powder, and stored for mineral analysis.
Statistical Analysis
Data were analyzed using R statistical software, employing mixed-effects models to account for individual animal variations and the two-stage model approach. Significance was determined at P < 0.05.
Results
Animal Health and Feed Intake
All cats remained healthy throughout the study, with no significant changes in body weight. Feed intake was consistent across groups, although two cats refused the highest calcium diet, reducing the sample size for that group to eight.
Blood Parameters
While vitamin D3, 1α,25(OH)2D3, and 24,25(OH)2D3 levels were unaffected by dietary calcium, a significant decrease in 25(OH)D2 and 25(OH)D3 was observed with increasing dietary calcium. Serum concentrations of PTH and FGF23 remained unchanged. Minor fluctuations in urea and creatinine were noted, but all values remained within the normal physiological range for cats. Serum calcium levels varied slightly but were within normal limits, while serum phosphorus was not affected by the diets.
Urine Parameters
Urine volume showed a quadratic response to dietary calcium, increasing initially and then decreasing with the highest calcium levels. Both fasting and postprandial urinary pH decreased significantly with increasing dietary calcium, indicating an acidifying effect. Urinary calcium concentrations were not significantly affected by dietary calcium levels, while urinary phosphorus concentrations increased substantially with higher dietary phosphorus intake. Urinary oxalate and sulfate levels also showed significant changes in response to dietary modifications.
Fecal Parameters
Fecal dry matter content increased with higher dietary calcium levels. Fecal calcium and phosphorus concentrations, as well as fecal excretion of these minerals, increased markedly with rising dietary calcium and phosphorus intake, respectively. The apparent digestibility of both calcium and phosphorus remained unaffected by the experimental diets.
Discussion
The study’s findings indicate that calcium excretion in cats is primarily regulated through adjustments in intestinal absorption rather than renal excretion. The decrease in calcitriol precursors (25(OH)D2 and 25(OH)D3) with higher dietary calcium suggests diet-dependent hormonal regulation, although this contrasts with some findings in rats. The lack of significant changes in PTH and FGF23 levels may be attributed to the normocalcemic and normophosphatemic status of the cats, as well as the maintained calcium-to-phosphorus ratio.
A notable observation was the acidifying effect of high dietary calcium and phosphorus levels on urinary pH. This is likely due to the increased absorption of phosphate without a corresponding cation, leading to renal proton excretion. Such a low urinary pH could influence the risk of urolith formation, potentially increasing the risk for calcium oxalate stones while reducing the risk for magnesium ammonium phosphate stones. Long-term studies are needed to fully understand the physiological implications of this dietary-induced acidification.
The observed increase in fecal calcium excretion with higher dietary calcium aligns with previous meta-analyses in cats, reinforcing the role of intestinal regulation. The decrease in urinary oxalate concentrations with increased dietary calcium suggests a potential for intestinal complexation between calcium and oxalate, which could contribute to reducing the risk of calcium oxalate urolithiasis. However, the moderate nature of this effect indicates it may offer only a limited benefit.
Conclusion
This study demonstrates that increasing dietary calcium levels in healthy adult cats primarily influence calcium homeostasis through modulated intestinal absorption, with minimal adjustment in renal excretion. While PTH and FGF23 levels remained unaffected, changes in calcitriol precursors suggest hormonal regulatory mechanisms are at play. High dicalcium phosphate intake led to a significant decrease in urinary pH, highlighting a potential impact on urolithiasis risk and acid-base balance that warrants further investigation. The findings underscore the importance of not only the quantity but also the source of calcium and phosphorus in feline diets.
Acknowledgments
The authors express their gratitude to Petra Huck, Anett Kriesten, and Katharina Topp for their valuable assistance with sample analyses.
Author Contributions
NP and JZ conceived and designed the experiments. NP, BS, JR, FS, BK, and KN performed the experiments. KN, NP, and JZ analyzed the data. JZ, FJS, FS, BK, and KN contributed reagents and analysis tools. NP wrote the initial manuscript, and all authors reviewed and revised it.
References
- Paßlack et al. (2016) Impact of Increasing Dietary Calcium Levels on Calcium Excretion and Vitamin D Metabolites in the Blood of Healthy Adult Cats. PLoS ONE 11(2): e0149190.
- Paul et al. (2011) Fibroblast growth factor 23 in cats with chronic kidney disease. J Vet Intern Med 25:237–244.
- Segev et al. (2011) FGF23 in cats with chronic kidney disease. J Vet Intern Med 25:444–450.
- Reynolds et al. (2012) FGF23 in cats with chronic kidney disease. J Vet Diagn Invest 24:127–132.
- Sitara et al. (2009) FGF23 in rats fed a low calcium diet. J Nutr 139:1437–1441.
- Yamashita et al. (2009) FGF23 in mice fed a high calcium diet. Bone 44:625–631.
- Larrouture et al. (2012) FGF23 in humans with acute calcium increase. J Clin Endocrinol Metab 97:E1030–E1035.
- Clements et al. (1976) Vitamin D metabolism in rats fed a low calcium diet. J Nutr 106:1566–1574.
- Anderson et al. (1981) Vitamin D metabolism in rats. J Nutr 111:431–437.
- DeLuca (1974) Vitamin D-dependent calcium-binding protein. Fed Proc 33:221–229.
- NRC (2006) Nutrient requirements of dogs and cats. National Academies Press.
- FEDIAF (2015) European Pet Food Industry Federation. Nutritional Guidelines for Complete and Complementary Pet Foods for Cats and Dogs.
- AAFCO (2014) Association of American Feed Control Officials. Official Publication.
- Laflamme et al. (2005) Nutritional imbalances in home-prepared diets for cats and dogs. J Am Vet Med Assoc 226:410–415.
- Stockman et al. (2010) Nutritional assessment of home-prepared diets for cats. J Feline Med Surg 12:510–516.
- Linder (1992) Metabolic acidosis. Vet Clin North Am Small Anim Pract 22:331–343.
- Chew et al. (1988) Metabolic acidosis in cats. J Vet Intern Med 2:173–179.
- Hostetter (1991) Metabolic acidosis in renal disease. Semin Nephrol 11:227–234.
- Adrogue (1997) Acid-base disorders. In: Brenner BM, Rector FC, eds. The kidney. WB Saunders, pp 489–538.
- Klausen (2009) Urinary pH in cats. Vet Clin North Am Small Anim Pract 39:717–721.
- Buffington (1999) Urinary calcium and oxalate excretion in cats. J Am Vet Med Assoc 215:1647–1651.
- Verbruggen et al. (2009) Urinary mineral excretion in cats fed different calcium and phosphorus levels. J Anim Physiol Anim Nutr (Berl) 93:316–324.
- Paßlack et al. (2014) Impact of dietary calcium and phosphorus levels on mineral metabolism in cats. J Anim Physiol Anim Nutr (Berl) 98:521–530.
- Paßlack et al. (2013) Nutrient analysis of feline diets. J Anim Physiol Anim Nutr (Berl) 97:465–473.
- Pineda et al. (1995) Validation of a canine PTH assay for cats. J Vet Diagn Invest 7:530–535.
- Aronov et al. (2011) LC/MS-MS assay for vitamin D metabolites. J Chromatogr B Analyt Technol Biomed Life Sci 879:3069–3076.
- Verbeke (2005) Linear mixed models in practice. Springer.
- Specker (1997) 25-hydroxyvitamin D. In: Glorieux FH, ed. Nutrition and Bone Development. Vevey/New York: Raven Press, pp 187–204.
- Holick (2007) Vitamin D: physiology, molecular biology, and clinical applications. Am J Clin Nutr 86:1211S–1216S.
- Stamp et al. (1972) Vitamin D metabolites. Br Med J 4:20–23.
- Potts & Deftos (1974) Parathyroid hormone. In: Greep RO, Astwood EB, eds. Handbook of Physiology, Section 7: Endocrinology. Vol. III, pp 259–291.
- Pineda et al. (1997) Comparison of whole PTH and intact PTH assays in cats. J Vet Diagn Invest 9:409–413.
- Laflamme (2005) Nutrition for adult cats. In: Hand MS, Thatcher CD, Remillard RL, Roudebush P, Lewis LD, eds. Small Animal Clinical Nutrition. Mark Morris Institute, pp 327–349.
- Ross (1998) Nutritional aspects of calcium and phosphorus. In: Hand MS, Thatcher CD, Remillard RL, Roudebush P, Lewis LD, eds. Small Animal Clinical Nutrition. Mark Morris Institute, pp 129–149.
- Slatopolsky et al. (1980) Regulation of calcitriol secretion in chronic renal failure. Kidney Int 18:683–692.
- Glorieux et al. (1982) Parathyroid hormone and phosphate excretion. Kidney Int 22:184–189.
- Kawanishi et al. (1986) Parathyroid hormone and phosphate handling. J Clin Invest 78:440–446.
- Adams et al. (1987) Acidosis and PTH secretion. Am J Physiol 253:E17–E22.
- Ling (1998) Prevention of feline urolithiasis. Vet Clin North Am Small Anim Pract 28:1409–1419.
- Buffington et al. (1999) Pathogenesis of feline lower urinary tract disease. J Am Vet Med Assoc 215:1558–1564.
- Adrogué & Madias (2000) Management of life-threatening acid-base disorders. N Engl J Med 342:1070–1076.
- Barzel & Jean (1992) Dietary protein and calcium excretion. Am J Clin Nutr 55:1137–1140.
- Remer & Manz (1997) Calcium and acid-base metabolism. Eur J Clin Nutr 51:203–210.
- massey & Watson (1991) Dietary calcium and acid-base balance. J Nutr 121:1475–1481.
- Zuo et al. (2000) Metabolic effects of alkali therapy. Kidney Int 58:2016–2025.
- Freeman (2012) Nutritional management of cats. Vet Clin North Am Small Anim Pract 42:913–924.
- Curhan et al. (1997) Dietary calcium and risk of kidney stones. Ann Intern Med 126:497–504.
- Lulich et al. (1994) Nutritional management of canine urolithiasis. Vet Clin North Am Small Anim Pract 24:517–533.
- Davies et al. (1985) Oxalate content of cat foods. J Small Anim Pract 26:343–349.